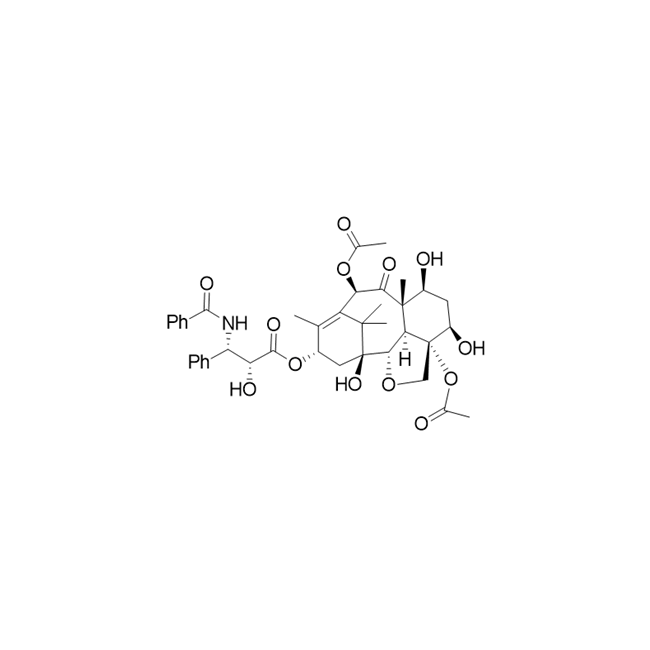
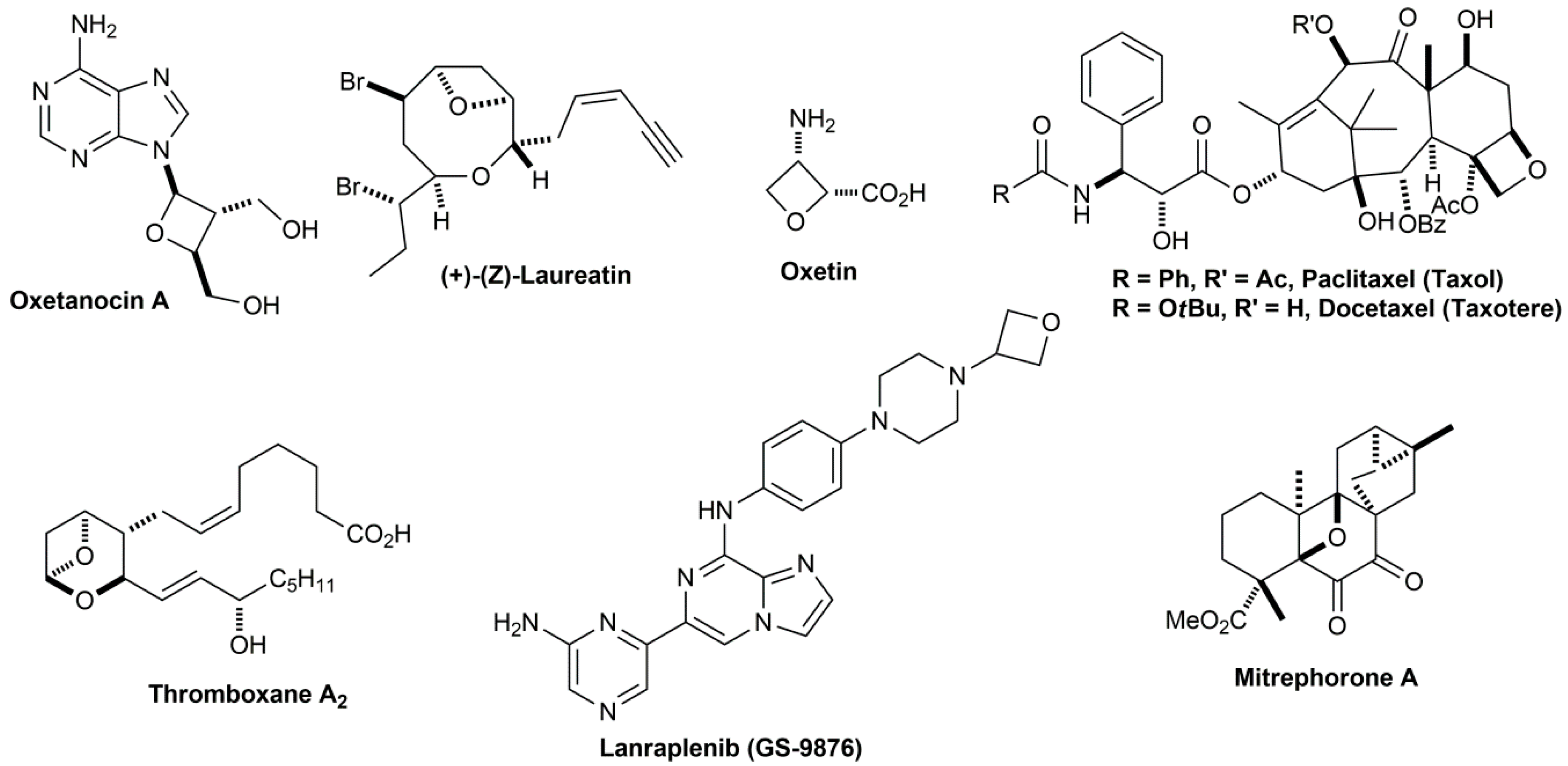
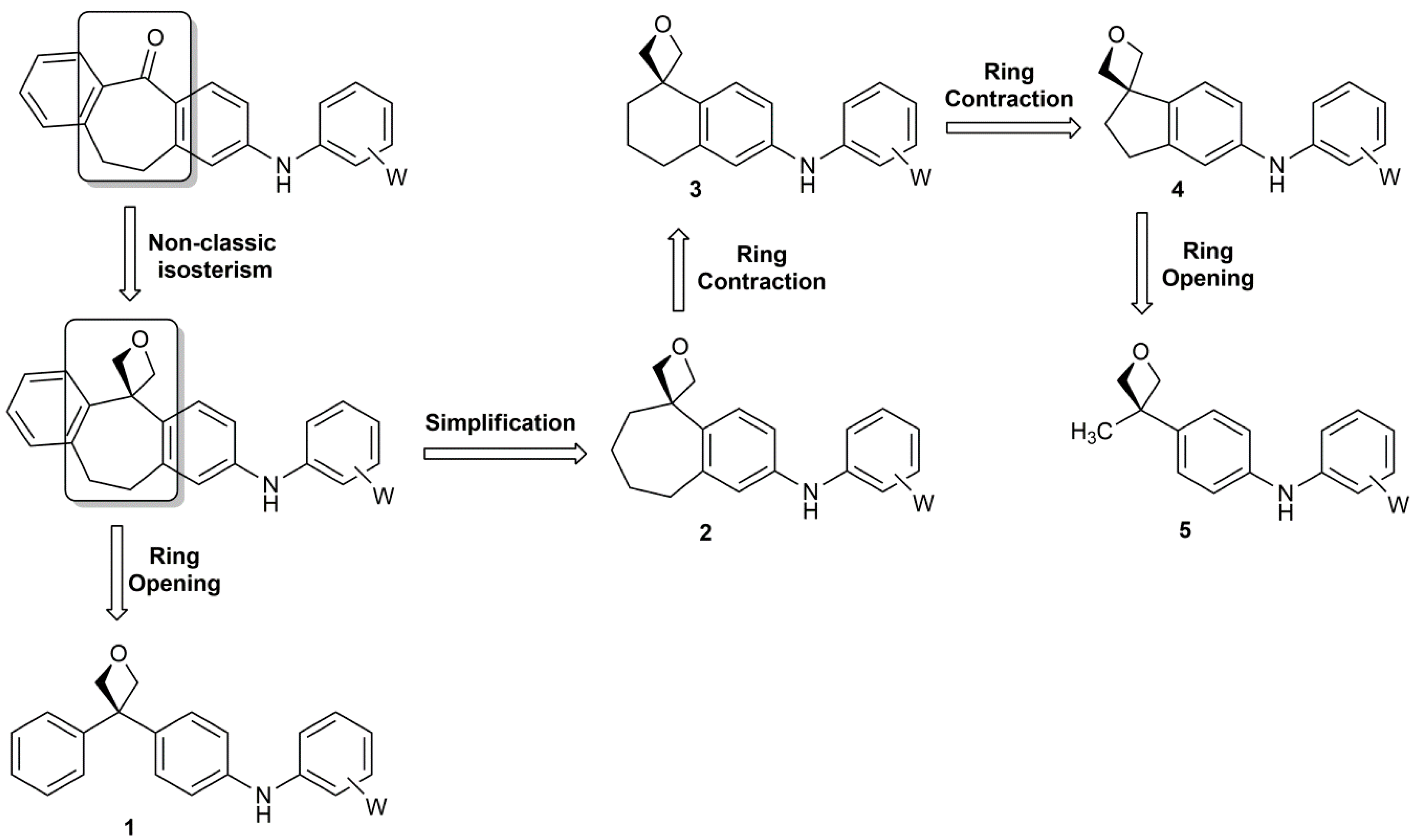
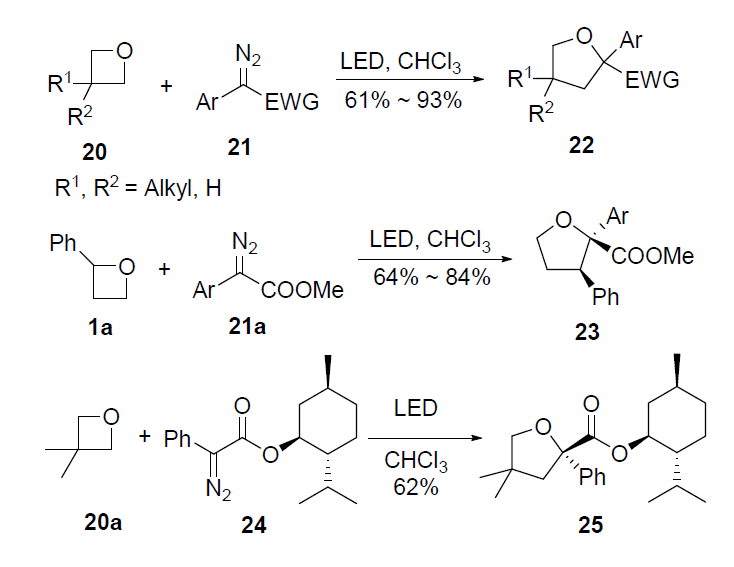
Ring opening polymerization of oxetane by the use of a montmorillonite clay as catalyst - ScienceDirect
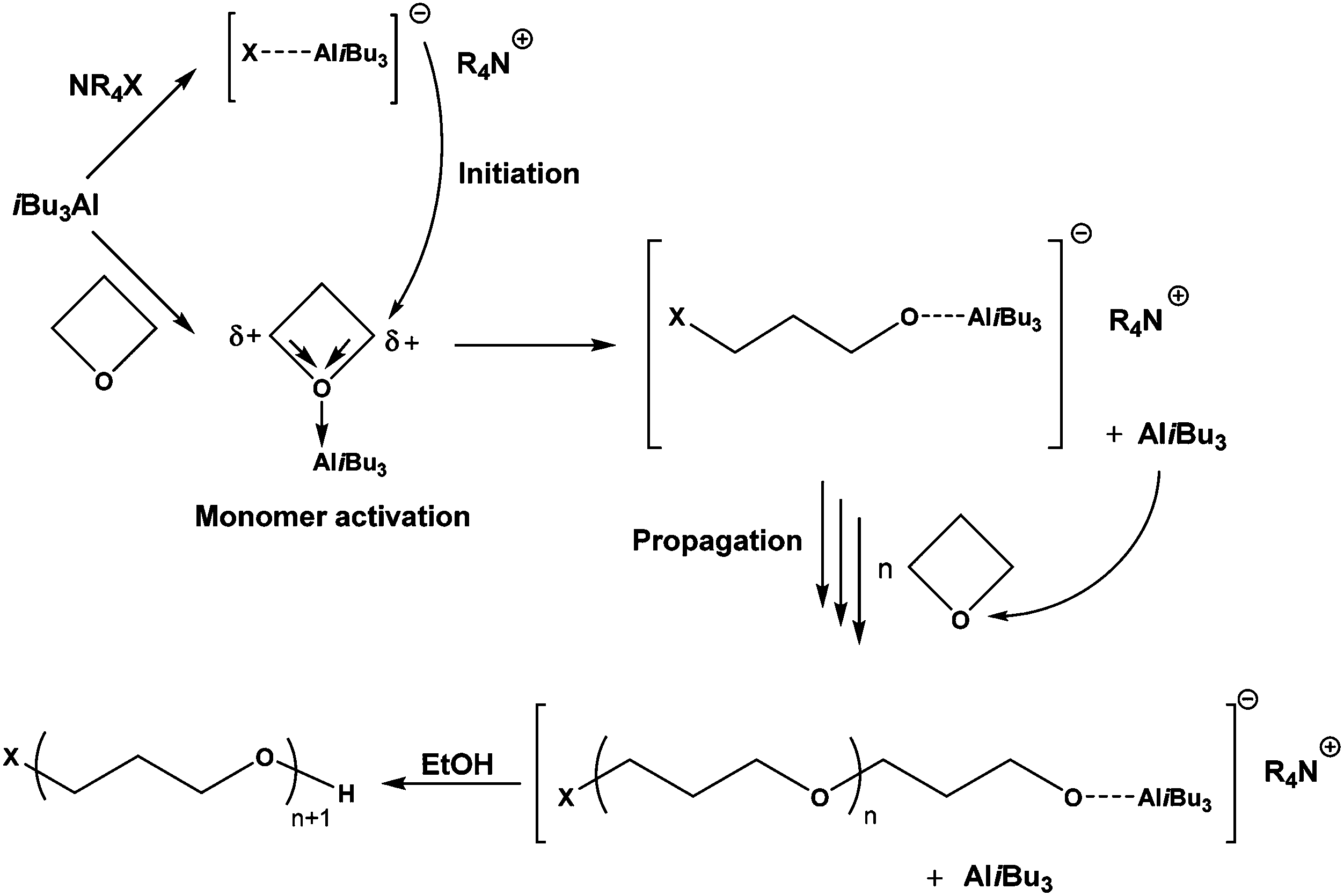
Anionic polymerization of activated oxetane and its copolymerization with ethylene oxide for the synthesis of amphiphilic block copolymers - Polymer Chemistry (RSC Publishing) DOI:10.1039/C8PY00307F

Radical Ring-Opening of Oxetanes Enabled by Co-Catalysis | Organic Chemistry | ChemRxiv | Cambridge Open Engage

Cationic ring opening polymerization of oxetane proceeds through the... | Download Scientific Diagram

Radical Ring-Opening of Oxetanes Enabled by Co-Catalysis | Organic Chemistry | ChemRxiv | Cambridge Open Engage
![PDF] Enantioselective Oxetane Ring Opening with Chloride: Unusual Use of Wet Molecular Sieves for the Controlled Release of HCl. | Semantic Scholar PDF] Enantioselective Oxetane Ring Opening with Chloride: Unusual Use of Wet Molecular Sieves for the Controlled Release of HCl. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0efaaee78d740e23578ee345b24b42d34f313217/3-Table4-1.png)
PDF] Enantioselective Oxetane Ring Opening with Chloride: Unusual Use of Wet Molecular Sieves for the Controlled Release of HCl. | Semantic Scholar
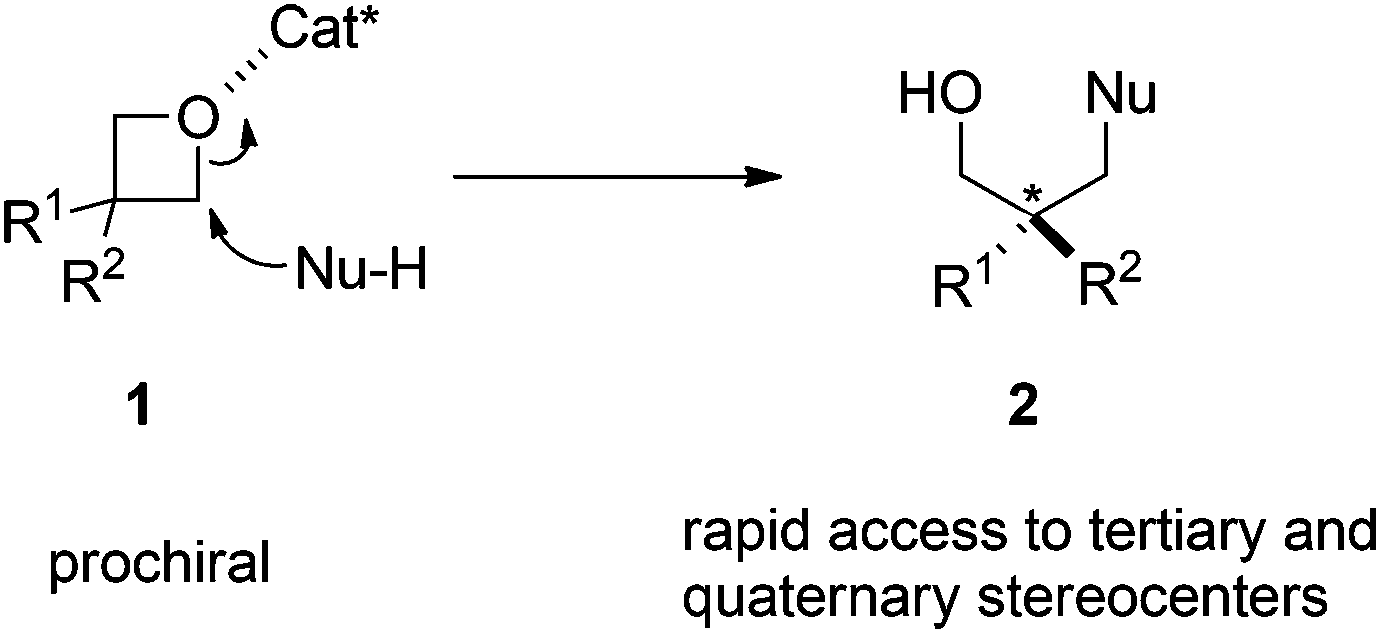
Catalytic asymmetric nucleophilic openings of 3-substituted oxetanes - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C4OB00920G

Mild C–C Bond Formation via Lewis Acid Catalyzed Oxetane Ring Opening with Soft Carbon Nucleophiles - Huang - 2021 - Angewandte Chemie International Edition - Wiley Online Library

Mild C–C Bond Formation via Lewis Acid Catalyzed Oxetane Ring Opening with Soft Carbon Nucleophiles - Huang - 2021 - Angewandte Chemie International Edition - Wiley Online Library

Enantioselective Oxetane Ring Opening with Chloride: Unusual Use of Wet Molecular Sieves for the Controlled Release of HCl - Yang - 2016 - Angewandte Chemie - Wiley Online Library
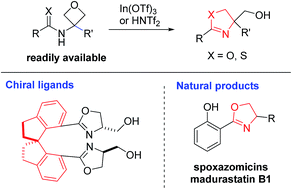
A mild catalytic synthesis of 2-oxazolines via oxetane ring-opening: rapid access to a diverse family of natural products - Chemical Science (RSC Publishing)
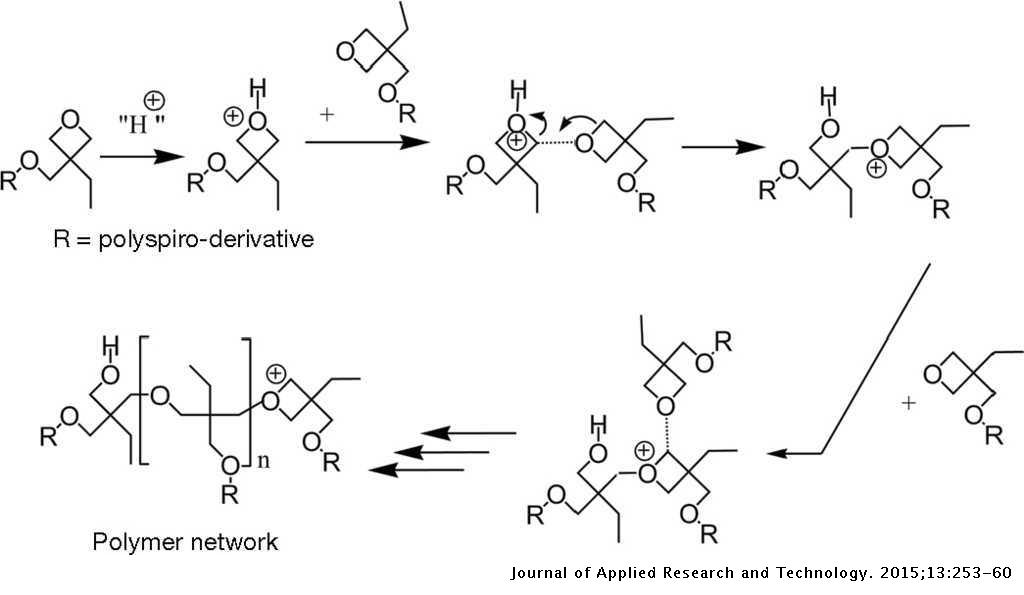
The I-V characteristics of organic hole-only devices based on crosslinked hole-transport layer | Journal of Applied Research and Technology. JART